When the ends of two wires from a circuit containing a battery and a lightbulb are placed into a beaker containing an aqueous solution the light bulb glows. The density of 200 mL of water was determined and then 20 grams of sugar was dissolved in the water.
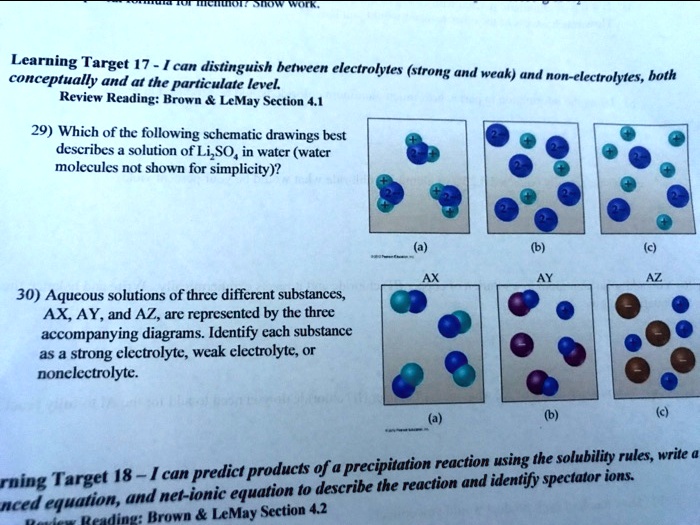
Solved Learning Target 17 Can Distinguish Between Electrolytes Strong And Weak And Conceptually And At The Particulate Level Non Electrolytes Hoth Review Reading Brown Lemay Section 4 1 29 Which Of The
As a consequence electrolyte solutions conduct electricity readily.
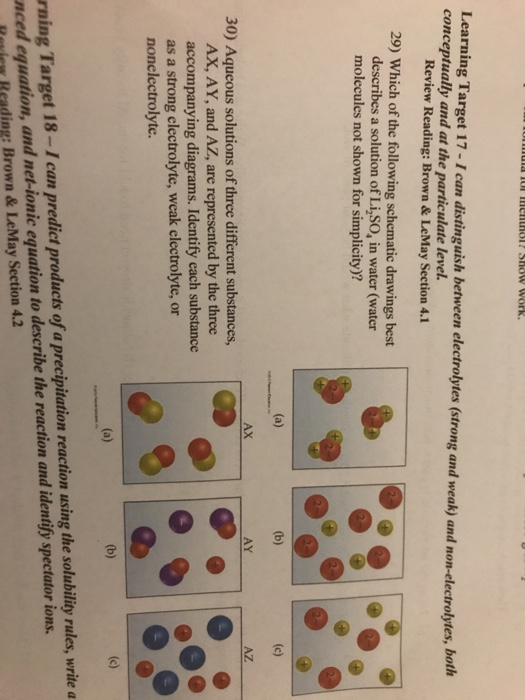
. Depends on the substances. Solutions Suspensions Colloids B. Solutions in which electric currents cannot run through are said to be.
Report Table 1 Conductivities and Electrolyte Classifications for Solutions Examined in Part A. Weak electrolytes include weak acids weak bases and a variety of other compounds. Rate of Dissolution E.
Ie there is virtually no undissociated form of the compound in solution. Other Apps - April 22 2022 11 2 Electrolytes Chemistry Post a Comment Read more Enter One Word That Describes the Culture of. There are virtually no molecules of a strong acid or base in solution only ions.
These ions can conduct electricity through the solution. The positive end of waters dipole. Experts are tested by Chegg as specialists in their subject area.
Most soluble salts acids and bases are electrolytes. A solute whose water solution conducts electricity is called a n Q. NH 3 - ammonia.
Electrolytes are salts or molecules which in solution ionise completely. Most compounds that contain nitrogen are weak electrolytes. Often the precipitate emerges as a suspension.
On the other hand the conduction of electricity is not possible in a nonelectrolyte solution. Solutions of nonelectrolytes such as ethanol do not contain dissolved ions and cannot conduct electricity. LONG QUIZ WEEK 9 6444 1930 1 The difference between the colligative properties of electrolyte and nonelectrolyte solutions is the breaking up of solute particles in a solution.
Net Ionic Equations J. In solution nonelectrolytes do not dissociate from ions. HF - hydrofluoric acid.
The student determined the density of the sugar-water solution and recorded the collected data in a table. Solutions of nonelectrolytes do not conduct electricity. Electrolytes disperse as molecules in solution.
Solution identity and concentration conductivity μ Scm electrolyte classification Deionized water 26 nonelectrolyte Tap water 805 weak 004 M acetic acid CH3COOH 305 weak 004 M sucrose C12H12O11 16 nonelectrolyte 004 M hydrochloric acid. Describe the qualities of ionic electrolytes. As electrolytes ionically bound substances act.
Strong electrolytes partially ionize in solution. We review their content and use your feedback to. The main difference between electrolytes and nonelectrolytes is that.
Who are the experts. Electrolytes are chemical compounds that can dissolve in water forming ions. CH 3 CO 2 H - acetic acid.
The conductivity of an electrolyte solution is related to the strength of. Compounds that dissolve in water. Which Best Describes Elevctrolytes and Nonelectrolytes in Solutions.
Nonelectrolytes are chemical compounds that do not conduct electricity when dissolved in water. This is because they do not form ions when dissolved in water. Electrolytes Nonelectrolytes D.
ELECTROLYTES are salts or molecules that ionize completely in solution. The emergence of the insoluble solid from solution is called precipitation. The ability to form an electric current.
Heats of Solution H. 1 blue 3 orange 2 yellow 4 red 27 Given the reaction. Concentration of Solutions Molarity Molality I.
A compound that does not conduct an electric current in either aqueous solution or in the molten state. Electrolyte A substance that dissolves in water to give a solution that conducts electric current. 1 Arrhenius acid and an electrolyte 2 Arrhenius acid and a nonelectrolyte 3 Arrhenius base and an electrolyte 4 Arrhenius base and a nonelectrolyte 26 According to Reference Table M what is the color of the indicator methyl orange in a solu-tion that has a pH of 2.
As a result electrolyte solutions readily conduct electricity. A compound that conducts an electric current when it is in an aqueous solution or melted. The sugar in this investigation is.
In chemistry a precipitate is an insoluble solid that emerges from a liquid solution. Which best describes a precipitate. Electrolytes are salts or molecules that desociates into its ions in solution as a result they conduct electricity while Non electrolyte s dont desociates into ions in solution and as a result dont conduct electricity Sugar solution is non ele.
H 2 O - water weakly dissociates in itself. A substance that when dissolves in water will product ions and conduct electricity non-electrolyte a substance that will not dissociate when dissolve in water strong electrolyte. Weak electrolytes only partially break into ions in water.
This is quantified and added to computation of concentration. Nonelectrolyte solutions do not therefore conduct electricity. This statement is not true because They disperse as ions not as molecules.
Electrolytes are the compounds that completely ionized into its ions in the water solvents whereas nonelectrolytes are the compounds that do not ionize into ions in the water solvents. Upon the dissociation of potassium chloride KCl the chloride ions Clwill interact with. An example of a nonelectrolyte is.
Even insoluble ionic compounds eg AgCl PbSO4 CaCO3 are strong electrolytes because the small amounts that do dissolve in water do so principally as ions. Also know what is a Nonelectrolyte. Log in to add comment.
The positive end of waters dipole. Yet covalently bound materials where. Some molecular substances are electrolytes.
Hope you get the answer report flag outlined. Compounds that dissolve by breaking into ions and conduct electricity in solution are known as electrolytes. The conduction of electricity is possible in an electrolyte solution.
All electrolytes are ionic substances. Solutions of electrolytes contain ions that permit the passage of electricity. Hence no electrolyte solutions do not conduct electricity.
NON ELECTROLYTES do not dissociate into ions in solution.

Solved C On Show Work Learning Target 17 I Can Chegg Com

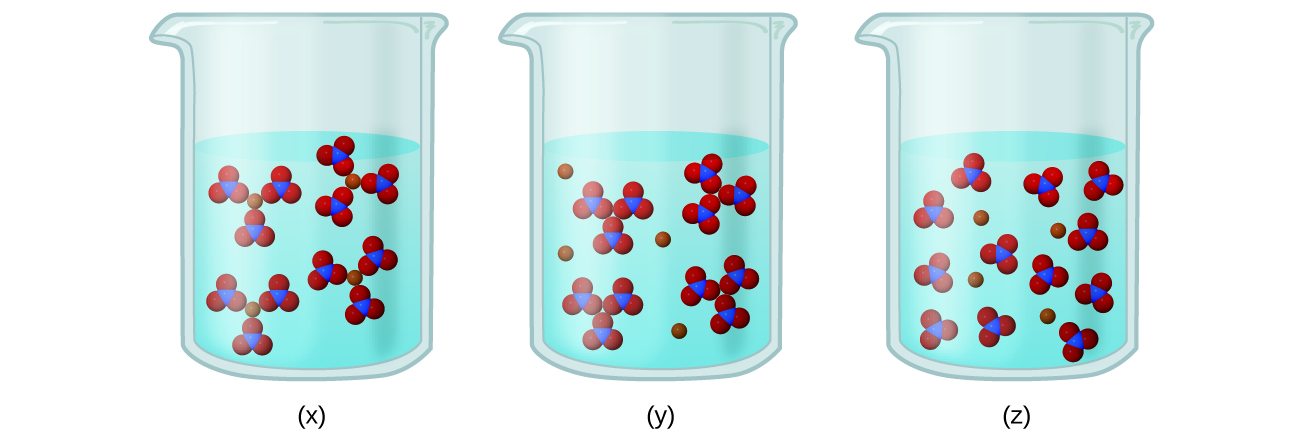
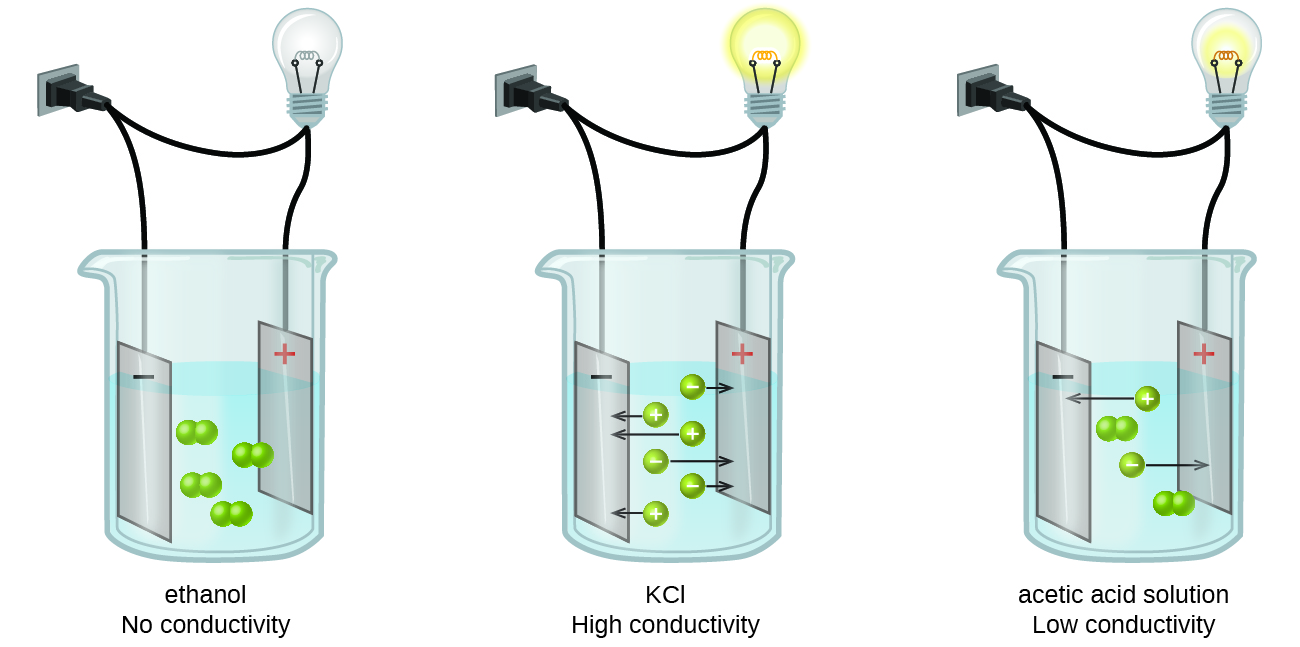
0 Comments